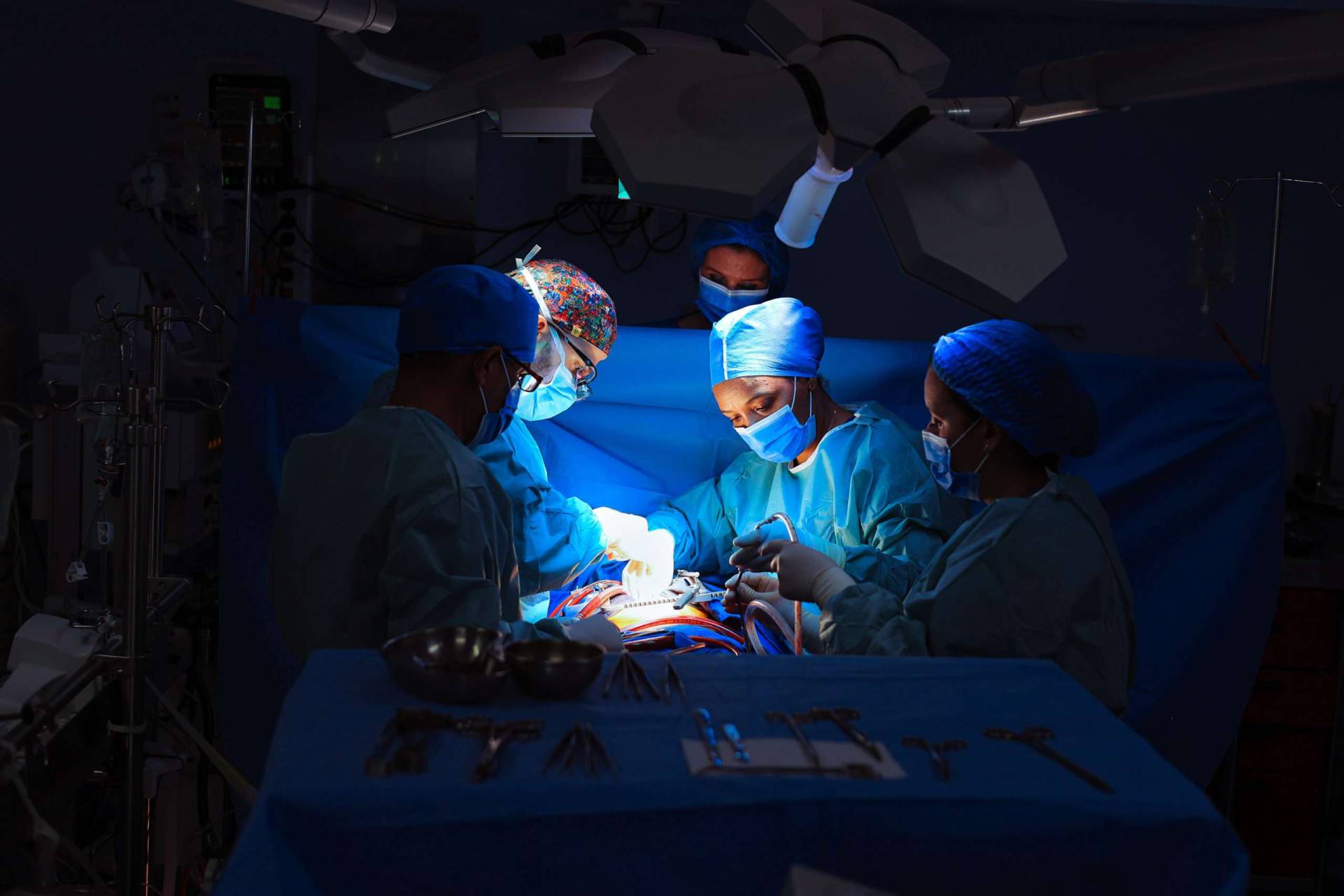
La clinique de Chirurgie Cardio-Vasculaire de l’Agdal a été fondée en 2005 par un groupe de chirurgiens et de réanimateurs dont deux anciens professeurs à la faculté de médecine et de pharmacie de Rabat, après une longue expérience de la chirurgie cardiaque à l’hôpital IBN SINA de Rabat.
Une équipe dévouée et humaine, avec une préoccupation constante : votre rétablissement et votre bien être.